In the dynamic landscape of life sciences, where
breakthroughs and innovations shape the future, maintaining robust quality
standards is paramount for sustainable growth. The integration of Quality Management Software (QMS) has become a transformative significant change,
ensuring organizations not only meet but exceed rigorous quality protocols.
In this article, we will explore the intricate realm of QMS
software, deciphering its key benefits, understanding its implications in life
sciences, and addressing potential drawbacks.
Let us embark on a comprehensive journey to unravel the
depths of QMS, navigating through its advantages, disadvantages, and the
pivotal role it plays in shaping the quality landscape of the life sciences
industry.
What is QMS in Life Sciences?
Quality Management System (QMS) in life sciences serves as
the linchpin that holds together the fabric of quality assurance and
compliance. It acts as the compass, guiding organizations through the
intricacies of ever-evolving regulatory requirements and industry standards.
QMS encompasses processes, procedures, and documentation
that collectively ensure product quality, safety, and efficacy. As life
sciences push the boundaries of innovation, a robust QMS becomes indispensable
for navigating the complex regulatory landscape with finesse and precision.
Key Benefits of a Quality Management System (QMS)
Streamlined Compliance for Unmatched Regulatory Adherence
Ensuring compliance with ever-evolving regulations is a
perpetual challenge in life sciences. A well-implemented QMS acts as an
impenetrable shield, streamlining compliance efforts with unmatched precision.
It provides a structured framework for documentation,
audits, and corrective actions, significantly reducing the burden of compliance
management.
In parallel, the integration of energy
management and automation further fortifies organizational resilience,
ensuring that processes not only meet regulatory standards but also contribute
to sustainable and efficient operations.
This integrated approach aligns seamlessly with the
intricate demands of the life sciences industry, where precision, compliance,
and resource efficiency converge for optimal outcomes.
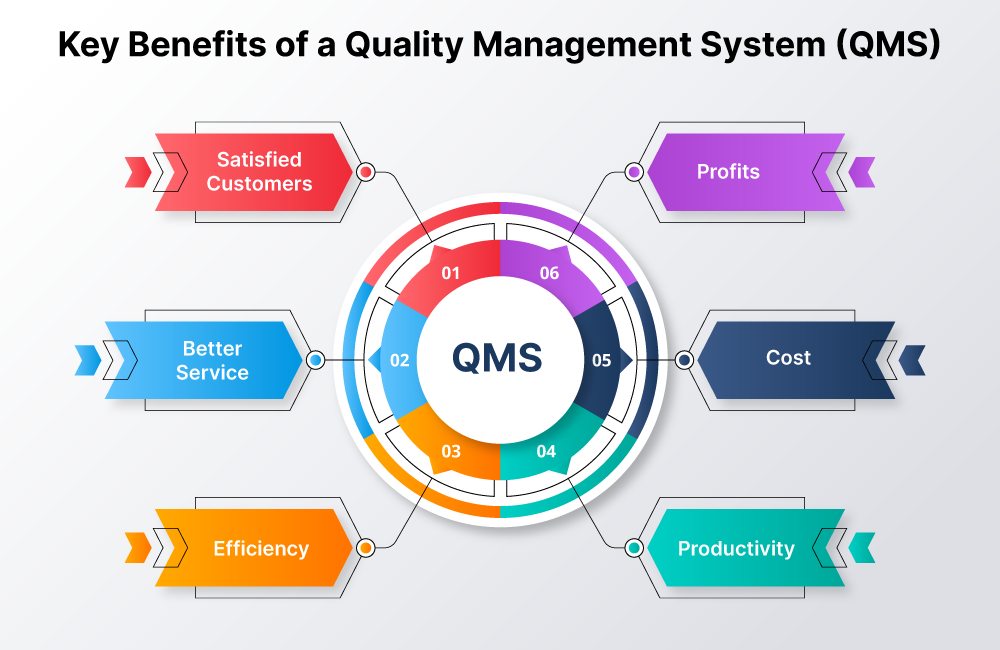
Enhanced Productivity: Empowering Innovations
Efficiency is the lifeblood of any industry, and life
sciences are no exception. QMS software optimizes processes, eliminating
redundancies and bottlenecks.
By fostering a culture of continuous improvement, it
enhances overall productivity, allowing organizations to focus on innovation
rather than grappling with operational inefficiencies.
Risk Mitigation: Initiative-taking Safeguarding
In the delicate realm of life sciences, where precision is
non-negotiable, the stakes are high. QMS does not just manage quality; it
proactively mitigates risks.
Through robust risk assessment and management modules, it
identifies potential hazards early on, enabling organizations to take initiative-taking
measures to safeguard the integrity of products and processes.
Improved Traceability: From Raw Materials to End Product
Traceability is crucial in the life sciences supply chain.
QMS facilitates end-to-end traceability, from the sourcing of raw materials to
the delivery of the final product. This not only ensures the quality of the product
but also expedites recalls and investigations if issues arise, minimizing
potential damage and reinforcing accountability.
Customer Satisfaction: Building Trust Through Quality
Ultimately, the goal of life sciences is to improve human
well-being. A QMS contributes to this noble pursuit by ensuring the delivery of
safe and effective products. This, in turn, enhances customer satisfaction and
fosters trust in the brand a priceless asset in an industry where reputation is
paramount.
Understanding EQMS Software: Navigating the Digital Evolution
Evolution Beyond Traditional QMS: Digital Transformation
The digital era has ushered in a new wave of Quality
Management Systems - EQMS (Electronic Quality Management System). EQMS software
goes beyond the limitations of traditional QMS, offering a dynamic and
integrated digital ecosystem for managing quality processes seamlessly.
From document control to training management, EQMS brings a
holistic and futuristic approach to quality management in the life sciences
sector.
This evolution mirrors the broader trend in organizational
efficiency, where EQMS aligns with the capabilities of innovative Business
Management Software, ensuring that quality processes not only adhere to
standards but also integrate seamlessly into the broader framework of efficient
and streamlined business operations.
This amalgamation of EQMS and Business Management Software
represents a change in basic assumptions in how organizations in the life
sciences sector approach quality and business process optimization.
Centralized Data Management: Breaking Down Silos
EQMS consolidates data into a centralized repository,
breaking down silos that often hinder collaboration. This not only ensures data
integrity but also facilitates real-time access to critical information,
empowering decision-makers in the life sciences domain to make informed choices
swiftly.
Disadvantages of QMS: Navigating the Pitfalls with Foresight
While the merits of QMS are evident, it is essential to
acknowledge the potential pitfalls:
Implementation Challenges: Embracing Change
Adopting a QMS, especially for organizations rooted in
traditional practices, can pose implementation challenges. Resistance to
change, training requirements, and initial setup costs are hurdles that need
careful navigation.
Embracing change with foresight is key to overcoming these
challenges and reaping the long-term benefits.
Resource Intensiveness: Strategic Resource Allocation
Maintaining a QMS demands resources, both in terms of
personnel and technology. The continuous monitoring, updating, and adherence to
evolving standards can be resource-intensive for some organizations.
Strategic resource allocation is vital to ensure the
sustainable implementation and success of a QMS in the dynamic landscape of
life sciences.
Overemphasis on Documentation: Balancing Act
In the pursuit of compliance, there is a risk of
overemphasizing documentation at the expense of practical application. Striking
the right balance is crucial to avoid bureaucracy that impedes agility. A QMS
should be a facilitator of streamlined processes, not a hindrance.
Conclusion: Navigating the Quality Landscape with Finesse
In conclusion, the integration of QMS, and specifically EQMS
software, is a transformative step for life sciences. The benefits in terms of
compliance, productivity, risk mitigation, and customer satisfaction are undeniable.
However, organizations must navigate the potential pitfalls
with foresight, ensuring a balanced approach that prioritizes practical
application over excessive documentation.
As the life sciences industry continues to evolve, the role
of QMS becomes increasingly pivotal. Embracing a robust QMS not only elevates
the quality standards but also positions organizations at the forefront of
innovation.
In a world where precision and reliability are
non-negotiable, QMS stands as the cornerstone, ensuring that life sciences
reach new heights of excellence, setting the stage for a future where quality is
not just a standard but a way of life.


